TPD Regulation Overview
-
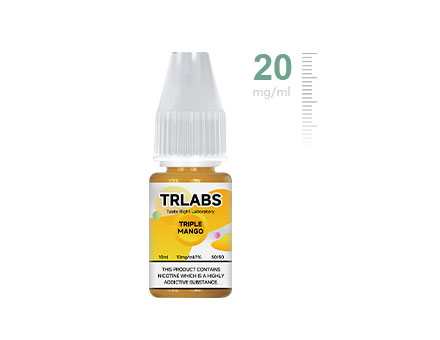 Nicotine Concentration Is Limited To 20mg/ml (2%)
Nicotine Concentration Is Limited To 20mg/ml (2%) -
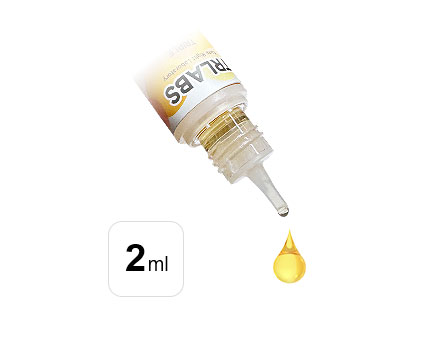 The Capacity Of The Pre Injection Atomization Chamber Cannot Exceed 2ml
The Capacity Of The Pre Injection Atomization Chamber Cannot Exceed 2ml -
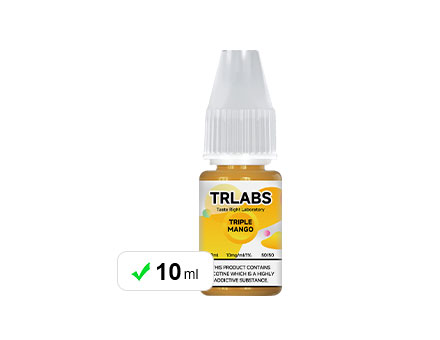 The Capacity Of The Atomized Liquid Bottle Cannot Exceed 10ml
The Capacity Of The Atomized Liquid Bottle Cannot Exceed 10ml -
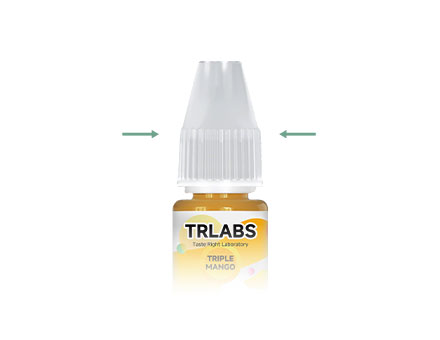 The Packaging Must Have A Child Lock Mechanism
The Packaging Must Have A Child Lock Mechanism -
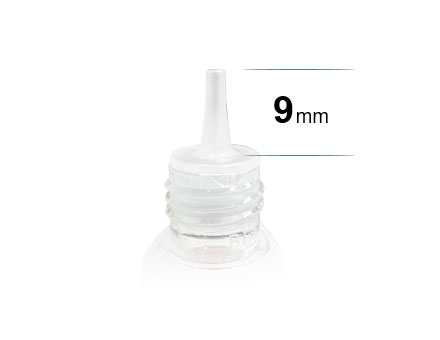 The Length Of The Dropper Should Be At Least 9mm
The Length Of The Dropper Should Be At Least 9mm -
 Drip Rate Controlled At No More Than 20 Drops Per Minute
Drip Rate Controlled At No More Than 20 Drops Per Minute
Packaging Compliance Requirements
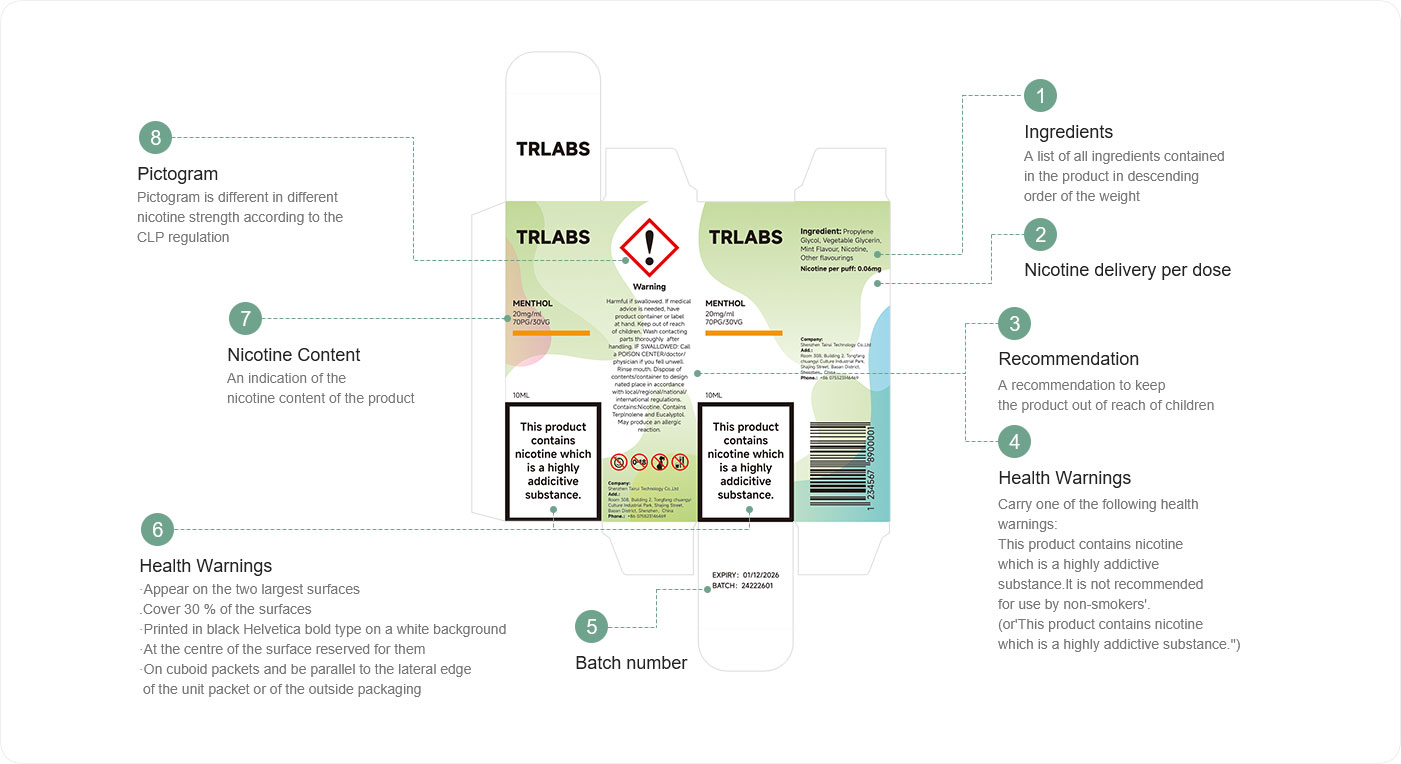
According To The Tobacco Products Directive (TPD), Bottled E-liquid Must Comply With A Series Of Safety Requirements And Ingredient Restrictions In The European Market. These Regulations Are Mainly Aimed At Ensuring The Safety Of Electronic Cigarette Liquids And Avoiding Harm To Consumer Health.
Toxic Substances: Any Ingredient That Is Harmful To Human Health Is Prohibited From Use. For Example, Certain Heavy Metals, Pesticide Residues, Carcinogens, Etc. Unclaimed Additives: All Ingredients In Electronic Cigarette Liquids Must Be Clearly Labeled And Additives That Have Not Been Scientifically Proven To Be Safe Must Not Be Used. High Concentration Nicotine: According To TPD Regulations, The Nicotine Concentration In Each Bottle Of Electronic Cigarette Liquid Cannot Exceed 20 Milligrams Per Milliliter, And The Maximum Capacity Of Each Bottle Is 10 Milliliters.
Some Essence And Additives: Some Essence Ingredients, Such As Some Substances With Potential Carcinogenic Risks (such As Formaldehyde, Acrolein, Etc.), Or Chemical Substances That May Have Adverse Effects On The Respiratory System, Are Prohibited. Ingredients That Can Cause Cardiovascular Problems, Such As Certain Types Of Chemicals That Can Increase The Burden On The Cardiovascular System.
Non Compliant Solvents With Quality And Safety Standards: All Solvents In Electronic Cigarette Liquids Must Comply With EU Food Or Pharmaceutical Safety Standards, And Toxic Or Unverified Solvents Cannot Be Used.
Other Requirements
Manufacturers And Importers Must Submit Technical Documentation For Each Product To The Relevant Authorities (MHRA, UK); A Comprehensive Toxicological Assessment Of Compounds And Their Emissions In Electronic Cigarette Liquids Is Required; The Main Material Used For Filling Bottles Should Be Recyclable Class 1 PET Plastic; Retailers And Manufacturers Must Share Market Data And Trends With Regulatory Authorities; TPD Requires Each Brand Of Electronic Cigarette Liquid To Submit A Detailed Ingredient List, Health Risk Assessment, And Product Packaging Design. They Must Also Undergo Standard Compliant Quality Control And Product Testing To Ensure Consumer Safety. Do You Want To Learn More About TPD Related Policies?



